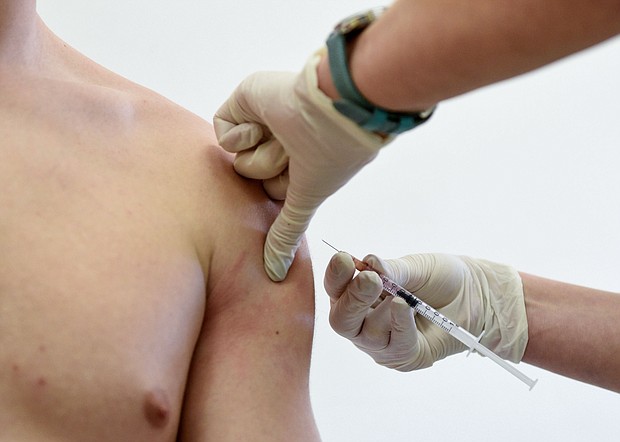
The US Food and Drug Administration is expected to grant emergency use authorization next week to Pfizer/BioNTech's coronavirus vaccine for teens and children ages 12 to 15.
Mandatory Credit: YURIY DYACHYSHYN/AFP/AFP via Getty Images
Stories this photo appears in:
Kids and the Covid-19 vaccine: A pediatrician answers safety questions
The US Food and Drug Administration is expected to grant emergency use authorization next week to Pfizer/BioNTech's coronavirus vaccine for teens and children ages 12 to 15.



