
A new gene therapy drug, the first in its class, was recommended for approval to the US Food and Drug Administration by an advisory committee on Wednesday, July 12, 2017. If approved by the FDA, the drug will be a new avenue for some leukemia patients whose first-line drugs have failed.
Stories this photo appears in:
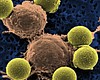
'Astounding' Second-chance Cancer Drug Heading for FDA Approval
A new gene therapy drug, the first in its class, was recommended for approval to the US Food and Drug Administration by an advisory committee on Wednesday. If approved by the FDA, the drug will be a new avenue for some leukemia patients whose first-line drugs have failed.



